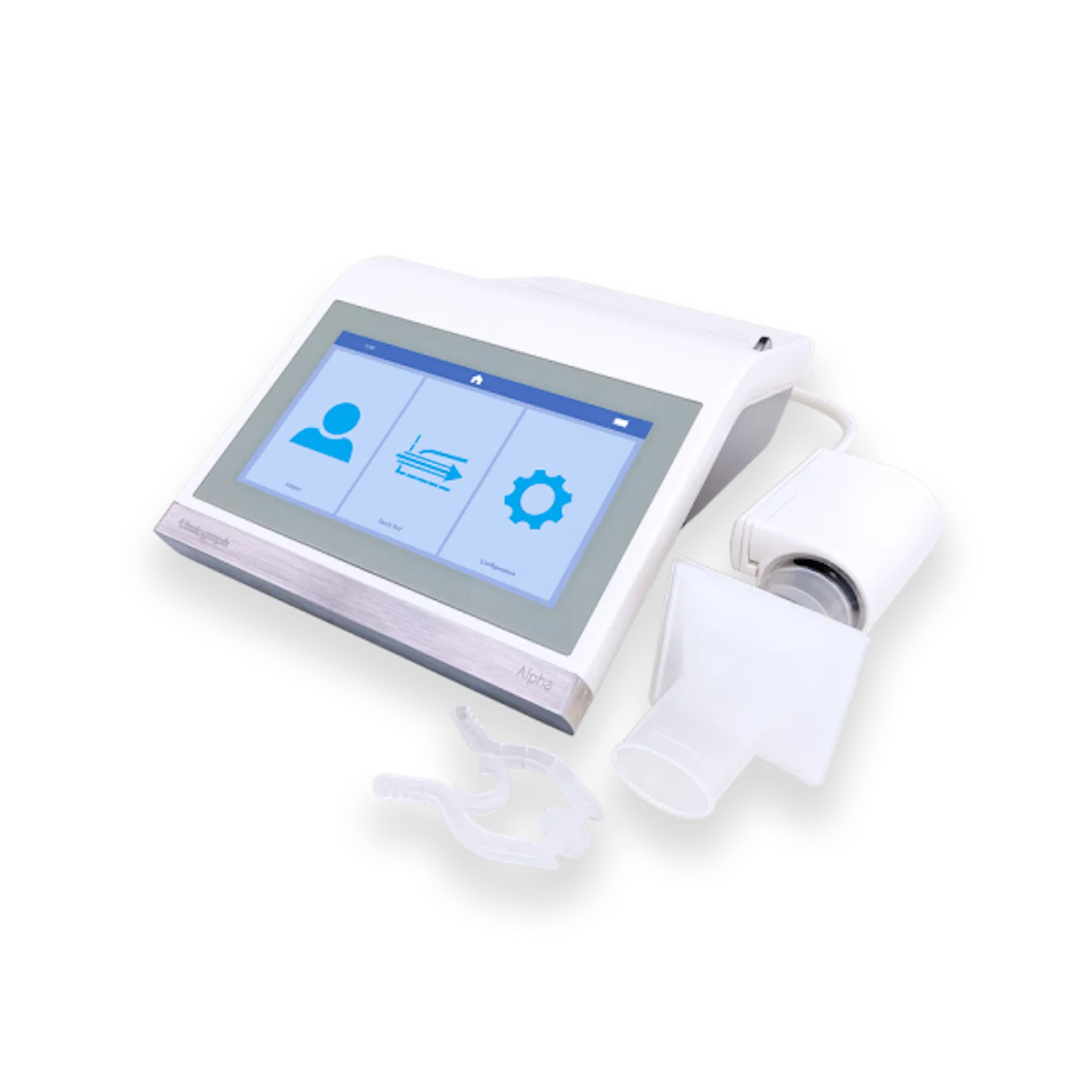
Capabilities
- Meets 2019 ATS/ERS standardization of spirometry update.
Supports full feature Spirometry Testing and Bronchodilator Responsiveness Testing with over 50 available parameters. - Utilising proven, highly accurate, robust, and stable flow measuring technology.
- Fast integral thermal printer – No PC required.
- Lowest running costs and environmentally friendly: no need for costly disposable sensors, turbines, spirettes or flow tubes.
- Save time and money on cleaning using Vitalograph BVFs with validated cross-contamination efficiency >99.999%, protecting the device, patient, and operator.
Instant quality feedback using the latest test/session acceptability, usability and repeatability criteria. - Automatic interpretation based on ATS/ERS and GOLD standards.
- Ensure accurate results with quick and easy calibration verification routines recommended by 2019 ATS/ERS spirometry guidelines.
- Fast evaluation of results using latest GLI spirometry predicted equations with LLN, %Pred and Z-scores. Other predicted equations are selectable.
- Direct connectivity with Vitalograph Device Studio to generate PDF reports and receive the latest firmware updates.
- Share test data easily with compatible Electronic Health Records (EHR) and Electronic Medical Records (EMR) systems through HL7, GDT.
Technical
- Model 6000
- Flow Detection Principal Fleisch flow technology
- Essential Performance Test Limits Flow accuracy ±10% or ±20 L/min, whichever is greater
- Volume Detection Flow integration sampling @ 100Hz
- Maximum Displayed Volume 10L
- Volume Accuracy Better than ±2.5%
- Voltage/Frequency Power supply
- Input 100-240V, 50/60Hz, Output 12V, 1.5A, Battery: 7.2V, 2.2Ahr
- Performance Standards met or exceeded by Vitalograph Alpha ATS/ERS 2019, ISO 23747:2015 and ISO26782:2009
- QA/GMP Standards EN ISO 13485, FDA 21 CFR 820, SOR/98-282 and JPAL, MDSAP
- Dimensions 204mm (length) x 253mm (width)
- Weight 1.5kg
- Power Supply 12V, 1.5A DC power supply
- 7.2V, 2.2AHr NiMH battery